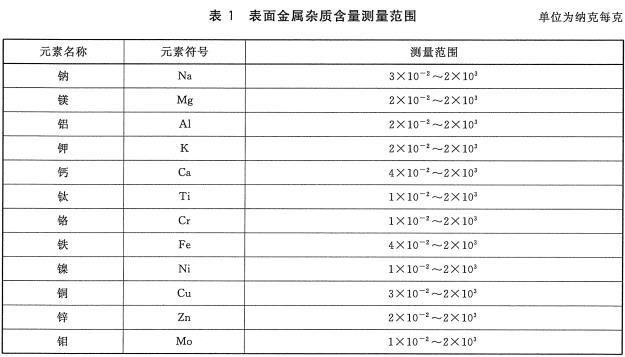
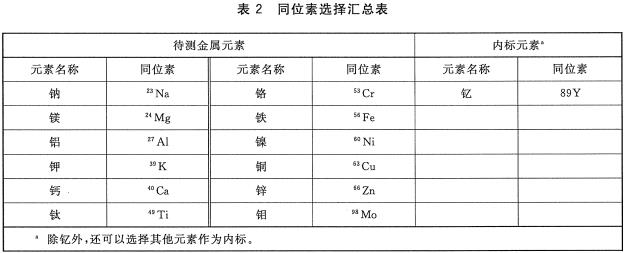
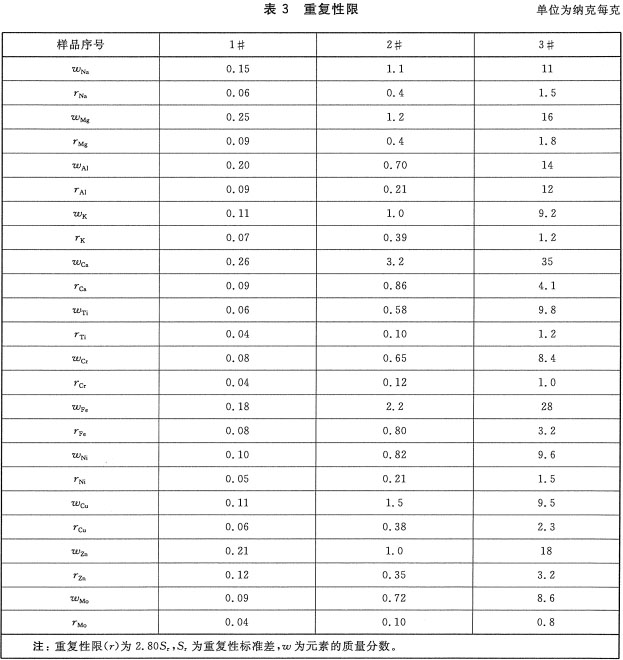
Foreword This standard was drafted in accordance with the rules given in GB/T 1.1-2009. Please note that some of the contents of this document may involve patents. The issuing agency of this document does not assume responsibility for identifying these patents. This standard was proposed and managed by the National Semiconductor Equipment and Materials Standardization Technical Committee (SAC/TC 203). This standard was drafted by: Information Industry Special Materials Quality Supervision and Inspection Center, China Electronics Standardization Institute, Jiangsu Zhongneng Silicon Technology Development Co., Ltd., National Electronic Function and Auxiliary Material Quality Supervision and Inspection Center, Tianjin Huanou Semiconductor Materials Technology Co., Ltd. the company. The main drafters of this standard: Yan Lianqing, Wang Hao, Xu Jing, Wang Xin, He Xiukun, Yan Huichuan, Feng Yabin, Lu Wenfeng, Zhang Xuegu. 1 Scope The current standard specifies the method of using ICP-MS to determine the content of trace metal impurities on the surface of silicon materials for photovoltaic cells. This standard applies to the measurement of trace metal impurities such as sodium, magnesium, aluminum, potassium, calcium, zinc, chromium, iron, nickel, copper, zinc and aluminum on the surface of silicon materials for photovoltaic cells. The measurement range of each element is shown in Table 1. 2 normative references The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this document. For undated references, the latest version (including all amendments) applies to this document. GB/T 25915.1-2010 Cleanrooms and related controlled environments - Part 1: Air cleanliness levels 3 method summary The sample was leached with a mixture of nitric acid, hydrofluoric acid, hydrogen peroxide and water for a certain period of time. After the sample was taken out, the leaching solution was heated and evaporated to dryness, and the silicon in the solution was volatilized as SiF4. Then, the residue was dissolved with nitric acid solution, and the content of the metal element to be analyzed in the solution was determined by an inductively coupled plasma mass spectrometer (ICP-MS) after determining the volume with ultrapure water. 4 Interference factors 4.1 Laboratory cleanliness, container and instrumentation The cleanliness of the sample introduction system, the purity of reagents and water, and the operation process directly affect the accuracy of the measurement results and should be strictly controlled. 4.2 Diatomic ions, polyatomic ions, matrix effects, background noise, inter-element interference, cross-contamination, and instrument drift can affect measurement results. 4.3 Due to the uneven distribution of metal impurities on the surface of the silicon material, the selection of the sample will affect the evaluation results of metal impurity content on the product surface, so the sample taken should be representative. 4.4 If the sample contains high levels of impurities, inductively coupled plasma atomic emission spectroscopy may be used to determine the concentration of the sample so that the instrument is not contaminated and the detection limits and accuracy of the instrument are affected. 5 Reagents 5.1 Ultrapure water: The resistivity is greater than 18.2 MΩ·cm, and the content of each metal impurity is less than 20 ng/L. 5.2 nitric acid: mass fraction "%, each metal impurity content is less than 10ng/L. 5.3 Hydrofluoric acid: Mass fraction 4800, each metal impurity content is less than 10ng/L. 5.4 Hydrogen peroxide: With a mass fraction of 3,000, the content of each metal impurity is less than 10ng/L. 5.5 Standard storage solution: The concentrations of sodium, magnesium, aluminum, potassium, calcium, Chromium, chromium, iron, nickel, copper, zinc, molybdenum, and memium are all 1 mg/mL, and certified reference materials traceable at home and abroad can be used. 5.6 Nitric acid solution: The volume ratio of nitric acid (see 5.2) and ultrapure water (see 5.1) is VHNO3:VH2 0-1:19. 5.7 Extraction solution: The volume ratio of nitric acid (see 5.2), hydrofluoric acid (see 5.3), hydrogen peroxide (see 5.4) and ultrapure water (see 5.1) is VHN03:VHF:VH202:VH2O=1:1: 1:50. 6 Instruments and Equipment 6.1 Inductively Coupled Plasma Mass Spectrometry. 6.2 analytical balance: The amount of sense is 0.01g. 6.3 fume hood. 6.4 Vessels: The vessels used shall be made of materials resistant to hydrofluoric acid corrosion and cleaning, such as polytetrafluoroethylene (PTFE) or perfluoroalkoxy (PFA). 6.5 hot plate. 7 Environmental conditions 7.1 The temperature is 18°C ​​to 25°C. 7.2 Relative humidity should not exceed 65%. 7.3 Cleanliness should be better than Class 6 clean room requirements as defined in GB/T 25915.1-2010. 8 sample preparation 8.1 Sampling shall be carried out in a clean room. A bag of no less than 5 kg of sample shall be selected from a batch of products for sampling, and it shall be assumed that the analyzed surface metal content represents the batch of samples. If samples are collected from different locations, the sample should be sealed and sent to the laboratory to avoid contamination of the sample during transfer. Staining should be strictly avoided during sampling. 8.2 In order to ensure the consistency of the analysis and comparison of laboratory analysis values, for a bulk sample, a standard weight or volume should be determined; for arbitration, six samples should be taken, each measuring approximately 3cm x 3cm x 3cm. The weight is about 50g, the total weight of the sample is about 300g, and at least 3 of the 6 samples have a growing outer surface. 9 test steps 9.1 sample amount Weigh a certain amount of sample (if the sample volume allows, we should weigh about 50g), accurate to 0.01g. 9.2 Measurements Three measurements were performed independently, with at least one sample with a growing outer surface, and the arithmetic mean of the three measurements was taken. 9.3 Blank test Test the blank with the sample. 9.4 Preparation of standard solution 9.4.1 Preparation of Mixed Element Standard Solutions Standard dilutions of sodium, magnesium, aluminum, potassium, calcium, zinc, chromium, iron, nickel, copper, zinc, and zinc (see 5.5) are serially diluted (maintaining the appropriate acidity during dilution) and are formulated as mixed elements. Standard solution, the concentration of each element is 1 μg/mL. 9.4.2 Preparation of Standard Series Working Solutions Add 5 μL, 20 μL, 50 μL, 100 μL, 200 μL of mixed element standard solution (see 9.4.1) to each of the 5 clean 100 mL volumetric flasks, and then add 40 mL of the nitric acid solution (see 5.6), using ultrapure water ( See 5.1) Constant volume. The concentrations of sodium, magnesium, aluminum, calcium, zinc, potassium, chromium, iron, nickel, copper, zinc, and platinum in this standard series of solutions were 0 μg/L, O.20 μg/L, O.50 μg/L, and 1.0 μg, respectively. /L, 2.0 μg/L. The prepared standard working solution concentration should be as close as possible to the concentration of the analyte in the sample solution. 9.4.3 Preparation of nail standard solution Pipette 100 μL of the standard stock solution (see 5.5) into a 100 mL volumetric flask, add 40 mL of nitric acid solution (see 5.6), dilute to volume with ultrapure water (see 5.1), shake, and the standard solution concentration is 1 μg. /mL. 9.4.4 Preparation of standard working solution Pipette 200 μL of the standard solution (see 9.4.3) into a 100 mL volumetric flask, add 40 mL of nitric acid solution (see 5.6), dilute to the mark with ultrapure water (see 5.1), shake well, and use this standard working solution concentration. It is 2 μg/L. 9.5 Preparation of sample solution Place the sample in a clean, open vessel with a suitable volume, add an appropriate amount of the leaching solution (see 5.7) and allow the sample to fully immerse the leaching solution (see 5.7), cover with a watch glass, and store at 70 °C on a hot plate heated for 60min, after cooling at room temperature, remove the sample with a clip, and rinse the surface of the sample with ultrapure water (see 5.1). The eluent is collected in an open container. The open vessel containing the leaching solution was heated to dryness on a 150°C hot plate. Remove the open container, cover with a watch glass, and after cooling at room temperature, add 4 mL of nitric acid solution (see 5.6) and shake well to completely dissolve the residue. Transfer the solution to a 10 mL volumetric flask, dilute to volume with ultrapure water (see 5.1), shake well and prepare for ICP-MS determination. 9.6 Instrument Analysis 9.6.1 Instrument Preparation Pre-test inductance plasma mass spectrometers need to set the appropriate operating conditions and tune to achieve the best test conditions. 9.6.2 Isotope Selection The choice of isotopes for each element to be analyzed and the internal standard element in the sample should be performed according to Table 2. 9.6.3 Analysis The blank solution, sample solution (see 9.5) and standard series working solution (see 9.4.2) were analyzed on an inductively coupled plasma mass spectrometer, and the standard working solution was used as an internal standard and corrected with an internal standard method. The ratio of the signal of each element in the standard series working solution to the signal of the internal standard element is the ordinate, and the calibration curve is made using the concentration of each element in the standard series working solution as the abscissa. The instrument automatically gives the blank solution and the sample solution (see 9.5). The concentration of each element in the test. According to the actual situation, other methods can also be used for quantitative analysis. 10 result processing Calculate the mass fraction of each metal impurity in the sample according to equation (1): 11 Precision Two independent test results are obtained under reproducibility conditions. Within the mean values ​​given below, the absolute difference between these two measurements does not exceed the repeatability limit (r), which exceeds the limit of repeatability (r). Not more than 5%, the repeatability limit (r) is obtained by linear interpolation according to the data in Table 3. 12 Quality Assurance and Control During the inspection, the control sample is applied to check the process. When the process fails, the cause should be found out. After correcting the error, it is checked again. 13 Report At a minimum, the report should contain the following: a) Sample delivery unit and sample delivery date; b) sample name, specification and number; c) sample status description; d) sample storage and transportation; e) the instrument model; f) measuring environment; g) measurement results; h) Operator, measurement date, measurement unit. Precision CNC machined stainless steel parts are becoming a choice for many industries due to its desirable physical properties! Stainless Steel is one of the most popular industrial alloys for many CNC machining projects, due to its excellent physical properties. The below benefits have made Stainless Steel parts and products a viable choice for many industries and applications, though they are particularly favored in the medical, automotive, aerospace, healthcare and consumer electronics spheres. While the best and fastest way to make Stainless Steel parts is CNC machining especially CNC milling with stainless steel reach far and wide. Stainless Steel Cnc Part,Stainless Steel Machine Parts,Stainless Steel Precise Part,Stainless Steel Cnc Part For Sale Taizhou TS HARDWARE Co., Ltd , https://www.shuwengroup.com